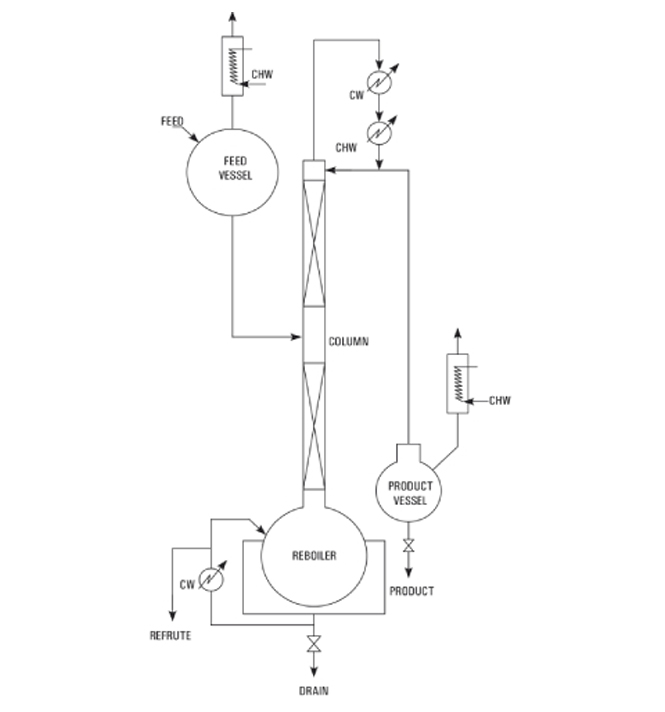
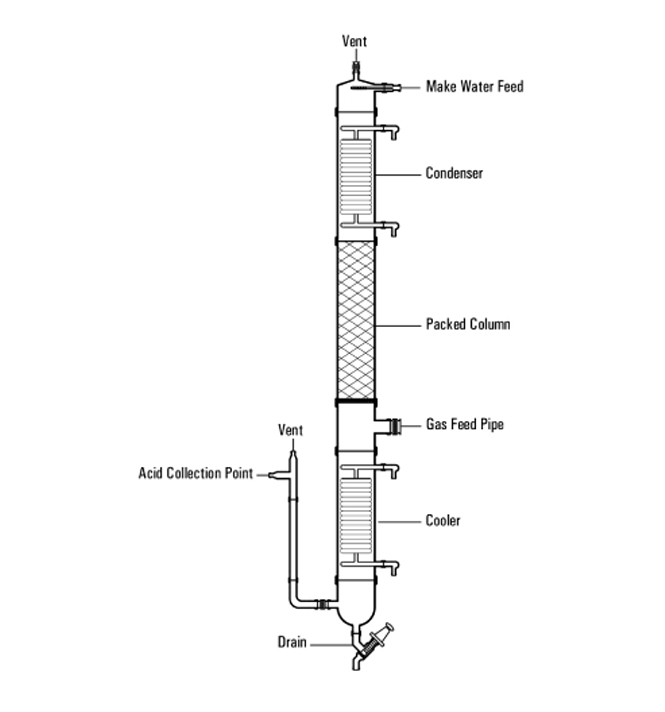
At Goel Impex, we pride ourselves on delivering the best industrial glassware solutions. Our continuous distillation system is designed to meet the various needs of industries seeking efficient, reliable, and customizable distillation processes.
What is a Continuous Distillation System?
A continuous distillation system is a highly efficient process that separates mixtures into parts based on different boiling points. Unlike batch distillation, continuous distillation operates non-stop, allowing for a consistent output of high-purity products.
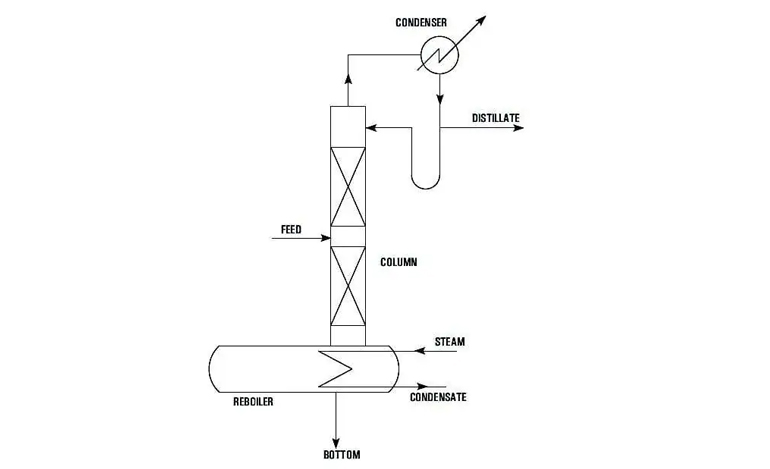
The constraints of batch distillations are readily overcome in a continuous distillation system, as seen in Figure 2, which depicts a typical fractionating unit consisting of ratification and stripping sections.
The feed is continually supplied into the column, with top and bottom products withdrawn. The process takes care of itself by keeping the feed and utility flow rates constant.
However, more columns are required when more than two products are wanted, such as in multicomponent systems, because each column can only produce two products.
In a multicomponent system, each column produces only one relatively pure product. The remaining components are put into a second column, producing one relatively pure product.
Column addition continues until the system becomes binary, with both components separated in the final column. A fundamental principle to remember is that a total of n-1 fractionators is necessary to separate a system with n components completely.
The relative volatility of each component in the feedstock determines which of two products in a column will be obtained in relatively pure form.
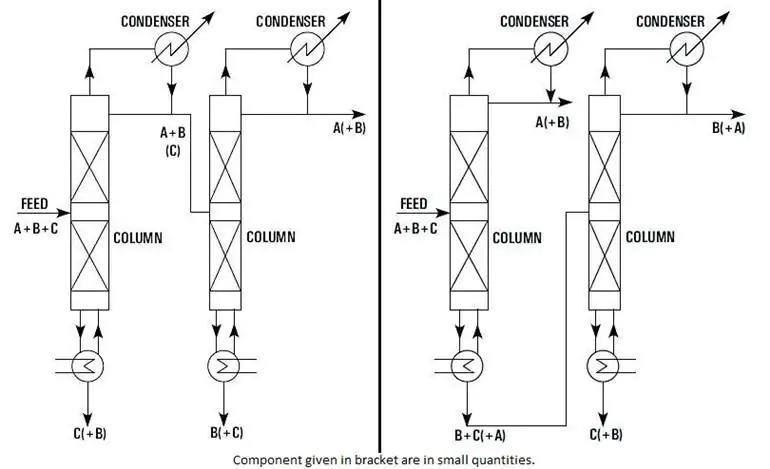
Consider a ternary solution made of components A, B, and C, with A being the most volatile. One of the strategies indicated in Figure 3 can be applied to obtain three chemicals in basically pure form.
The relative difficulty of separation in each process determines which strategy is chosen, and the decision necessitates further in-depth analysis of continuous distillation system principles.
However, method (b) is commonly chosen because it only requires one vaporization of substance A.